Pancreatic ductal adenocarcinoma (PDAC), the most common type of pancreatic cancer, is projected to become the second leading cause of cancer death in the United States by 2030 (1-2). Despite recent advances in systemic chemotherapy for metastatic PDAC with approval of several chemotherapy regimens in the last decade, median overall survival remains poor for this disease (<1 year) and novel treatment strategies are needed (3-5). Although initial studies of single agent immunotherapy agents with checkpoint inhibitors in PDAC have been underwhelming, much promise and interest in novel targeted and immunotherapy strategies for PDAC remains given our emerging understanding of the role the immune system in pancreatic cancer (6).
Published studies from pancreatic cancer patients have shown that the presence of tumor-infiltrating lymphocytes (TILs) in surgical resection specimens is associated with improved prognosis among patients who undergo surgical resection of their early stage pancreas cancers (7, 8), suggesting these T cells may be eliminating some of the tumor. TIL therapy relies on the ability to isolate and expand tumor-reactive T cells from within the patient tumor, which is challenging to implement. We are taking the more direct approach of engineering patients’ T cells with CARs that directly bind to cancer cell surface proteins, carbohydrates, or glycolipids.
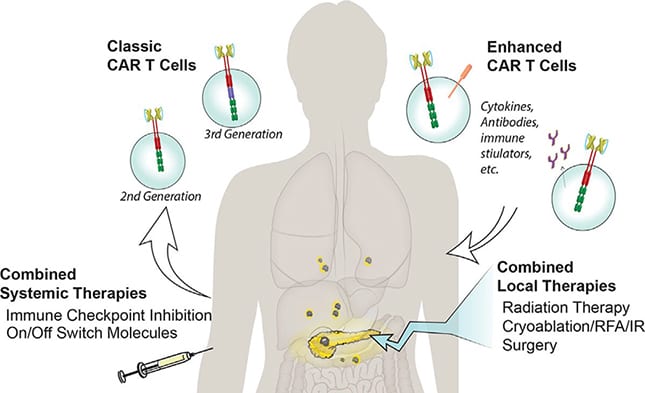
Although CAR T cell therapy had advanced quickly in the last decade, its use for pancreatic cancer remains in its infancy. Pancreatic cancer, in particular, provides barriers to successfully eliminating the tumor, such as a suppressive microenvironment and a dense stroma that impedes T cell penetration. We are currently testing CARs recognizing novel targets on pancreatic cancer, with various elements that enhance their efficacy. Thus far, studies in mice with pancreatic cancer are encouraging, and we are optimistic these approaches will translate to patients.
Featured image (top): CAR T cells communicate through thin finger-like projections in a coordinated attack against a pancreatic cancer cell.
References
- DeSelm CJ, Tano ZE, Varghese AM, Adusumilli PS. CAR T-cell therapy for pancreatic cancer. J Surg Oncol. 2017;116:63-74.
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-21.
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-25.
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-703.
- Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-57.
- Chang JH, Jiang Y, Pillarisetty VG. Role of immune cells in pancreatic cancer from bench to clinical application: An updated review. Medicine. 2016;95:e5541.
- Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26-31.
- Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914-23.